CBSE NCERT solutions for Class 10 Science Chapter 1 - Chemical Reactions and Equations in english: Looking best NCERT solutions for Class 10 Science? Want to increase your score in CBSE class 10 board?
 |
| CBSE NCERT solutions for Class 10 Science Chapter 1 - Chemical Reactions and Equations in english: TEGOFFICIAL.COM |
Get the book from the link below:
Increase your marks by writing better answers than other fellow students.
Increase your marks by writing better answers than other fellow students.
Solutions of In Text Questions of CBSE NCERT Class 10 Science Chapter 1 - Chemical Reactions and Equations
In text questions solutions of CBSE NCERT Class 10 Science on Page No: 6
Ques 1: Why should a magnesium ribbon be cleaned before it is burnt in air ?
Solution: Magnesium is very reactive metal. When it is stored, it reacts with oxygen to form a layer of magnesium oxide on its surface. This layer of magnesium oxide so formed is quite stable and prevents the further reaction of magnesium with oxygen. This magnesium ribbon is cleaned by used sand paper for removing this layer as a result of which the underlying metal can be exposed to air.
Ques 2: Write the balanced equation for the following chemical reactions.
(i) Hydrogen Chlorine → Hydrogen chloride
(ii) Barium chloride Aluminium sulphate → Barium sulphate Aluminium chloride
(iii) Sodium Water → Sodium hydroxide Hydrogen
Solution: The balanced equation for the above chemical reactions are as follows:
(i) H2 (g)+ Cl2 (g) → 2HCl(g)
(ii) 3BaCl2 (s) +Al2(SO4)3(s) → 3BaSO4(s) +2AlCl3(s)
(iii) 2Na(s) +2H2O(l) → 2NaOH(aq)+ H2(g)
Solution: Magnesium is very reactive metal. When it is stored, it reacts with oxygen to form a layer of magnesium oxide on its surface. This layer of magnesium oxide so formed is quite stable and prevents the further reaction of magnesium with oxygen. This magnesium ribbon is cleaned by used sand paper for removing this layer as a result of which the underlying metal can be exposed to air.
Ques 2: Write the balanced equation for the following chemical reactions.
(i) Hydrogen Chlorine → Hydrogen chloride
(ii) Barium chloride Aluminium sulphate → Barium sulphate Aluminium chloride
(iii) Sodium Water → Sodium hydroxide Hydrogen
Solution: The balanced equation for the above chemical reactions are as follows:
(i) H2 (g)+ Cl2 (g) → 2HCl(g)
(ii) 3BaCl2 (s) +Al2(SO4)3(s) → 3BaSO4(s) +2AlCl3(s)
(iii) 2Na(s) +2H2O(l) → 2NaOH(aq)+ H2(g)
Ques 3: Write a balanced chemical equation with state symbols for the following reactions.
(i) Solutions of barium chloride and sodium sulphate in water react to give insoluble barium sulphate and the solution of sodium chloride.
(ii) Sodium hydroxide solution (in water) reacts with hydrochloric acid solution (in water) to produce sodium chloride solution and water.
Solution: The balanced chemical equation with state symbols for the above reactions are:
(i) BaCl2 (aq) + Na2SO4 (aq) → BaSO4(s) + 2NaCl (aq)
(ii) NaOH (aq)+ HCl (aq) → NaCL (aq) + H2O (l)
In text questions solutions of CBSE NCERT Class 10 Science on Page No: 10
Ques 1: A solution of a substance ‘X’ is used for white washing.
(i) Name the substance ‘X’ and write its formula.
(ii) Write the reaction of the substance ‘X’ named in (i) above with water.
Solution: (i) The substance ‘X’ used for white washing is calcium oxide. Its chemical formula is CaO.
(ii) Calcium oxide reacts rapidly with water to form the calcium hydroxide (or slaked lime).
CaO (s) + H2O (l) → Ca(OH)2 (aq)
OR, Calcium Oxide (Quick Lime) + Water → Calcium Hydroxide (Slaked Lime)
Ques 2: Why is the amount of gas collected in one of the test tubes in Activity 1.7 double of the amount collected in the other? Name this gas.
Solution: Water (H2O) contains two parts of hydrogen and one part oxygen (2:1). As a result, during the electrolysis of water, the amount of hydrogen gas collected in one of the test tubes is double to that of the oxygen produced and collected in the other test tube.
In text questions solution of CBSE NCERT Class 10 Science on page no: 13
Ques 1: Why does the colour of copper sulphate solution change when an iron nail is dipped in it?
Solution: When an iron nail dipped in the copper sulphate (CuSO4) solution than the iron displaces copper from the copper sulphate because of the fact that iron is more reactive than copper. As a result, the colour of the copper sulphate solution changes.
The reaction involved in above reaction is:
Fe (s) +CuSO4 (aq) → FeSO4 (aq) +Cu (s)
Ques 2: Give an example of a double displacement reaction other than the one given in Activity 1.10.
Solution: An example of a double displacement reaction:
2KBr (aq) +BaI2 (aq) → 2KI (aq) +BaBr2 (aq)
Solution: When an iron nail dipped in the copper sulphate (CuSO4) solution than the iron displaces copper from the copper sulphate because of the fact that iron is more reactive than copper. As a result, the colour of the copper sulphate solution changes.
The reaction involved in above reaction is:
Fe (s) +CuSO4 (aq) → FeSO4 (aq) +Cu (s)
Ques 2: Give an example of a double displacement reaction other than the one given in Activity 1.10.
Solution: An example of a double displacement reaction:
2KBr (aq) +BaI2 (aq) → 2KI (aq) +BaBr2 (aq)
Ques 3: Identify the substances that are oxidised and the substances that are reduced in the following reactions.
(i) 4Na (s)+ O2 (g) → 2Na2O (s)
(ii) CuO (s) +H2 (g) → Cu (s)+ H2O (l)
Solution: The substances that are oxidised and the substances that are reduced in the above reactions are:
(i) Sodium (Na) is oxidised as it gains oxygen and oxygen gets reduced.
(ii) Copper oxide (CuO) is reduced to copper (Cu) while hydrogen (H2) gets oxidised to water (H2O).
(i) 4Na (s)+ O2 (g) → 2Na2O (s)
(ii) CuO (s) +H2 (g) → Cu (s)+ H2O (l)
Solution: The substances that are oxidised and the substances that are reduced in the above reactions are:
(i) Sodium (Na) is oxidised as it gains oxygen and oxygen gets reduced.
(ii) Copper oxide (CuO) is reduced to copper (Cu) while hydrogen (H2) gets oxidised to water (H2O).
Excercise questions solutions of CBSE NCERT Class 10 Science
Questions solutions of CBSE NCERT Class 10 Science Page No: 14
Ques 1: Which of the statements about the reaction below are incorrect?
2PbO (s) +C (s) → 2Pb (s)+ CO2 (g)
(a) Lead is getting reduced.
(b) Carbon dioxide is getting oxidised.
(c) Carbon is getting oxidised.
(d) Lead oxide is getting reduced.
(i) (a) and (b)
(ii) (a) and (c)
(iii) (a), (b) and (c)
(iv) all
Solution: The incorrect statements are:
(i) (a) and (b)
Ques 2: Fe2O3 + 2Al → Al2O3 + 2Fe
The above reaction is an example of a
(a) combination reaction.
(b) double displacement reaction.
(c) decomposition reaction.
(d) displacement reaction.
Solution: The above reaction is an example of option (d), i.e. displacement reaction.
2PbO (s) +C (s) → 2Pb (s)+ CO2 (g)
(a) Lead is getting reduced.
(b) Carbon dioxide is getting oxidised.
(c) Carbon is getting oxidised.
(d) Lead oxide is getting reduced.
(i) (a) and (b)
(ii) (a) and (c)
(iii) (a), (b) and (c)
(iv) all
Solution: The incorrect statements are:
(i) (a) and (b)
Ques 2: Fe2O3 + 2Al → Al2O3 + 2Fe
The above reaction is an example of a
(a) combination reaction.
(b) double displacement reaction.
(c) decomposition reaction.
(d) displacement reaction.
Solution: The above reaction is an example of option (d), i.e. displacement reaction.
Questions solutions of CBSE NCERT Class 10 Science on Page No: 15
Ques 3: What happens when dilute hydrochloric acid is added to iron filings? Tick the correct answer.
(a) Hydrogen gas and iron chloride are produced.
(b) Chlorine gas and iron hydroxide are produced.
(c) No reaction takes place.
(d) Iron salt and water are produced.
Solution: (a) The end products are: Hydrogen gas and iron chloride.
The reaction that takes place is: Fe + 2HCl → FeCl2 + H2
Ques 4: What is a balanced chemical equation? Why should chemical equations be balanced?
Solution: A chemical reaction that has an equal number of atoms of all the elements on both sides of the chemical equation (i.e. on the left side and right side of the arrow) is called a balanced chemical equation. It should be noted that chemical reaction should be balanced to follow law of conservation of mass.
Ques 5: Translate the following statements into chemical equations and then balance them.
(a) Hydrogen gas combines with nitrogen to form ammonia.
(b) Hydrogen sulphide gas burns in air to give water and sulphur dioxide.
(c) Barium chloride reacts with aluminium sulphate to give aluminium chloride and a precipitate of barium sulphate.
(d) Potassium metal reacts with water to give potassium hydroxide and hydrogen gas.
Solution: The balanced chemical reactions are:
(a) 3H2 (g) +N2 (g) → 2NH3 (g)
(b) 2H2S (g) +3O2 (g) → 2H2O(l) +2SO2 (g)
(c) 3BaCl2 (aq) +Al2(SO4)3 (aq) → 2AlCl3 (aq) +3BaSO4 (s)
(d) 2K (s) +2H2O (l) → 2KOH(aq) +H2 (g)
Ques 6: Balance the following chemical equations.
(i) HNO3 +Ca(OH)2 → Ca(NO3)2 +H2O
(ii) NaOH +H2SO4 → Na2SO4 +H2O
(iii) NaCl +AgNO3 → AgCl +NaNO3
(iv) BaCl2 +H2SO4 → BaSO4 +HCl
Solution: The balanced chemical reactions are:
(i) 2HNO3 +Ca(OH)2 → Ca(NO3)2 +2H2O
(ii) 2NaOH +H2SO4 → Na2SO4 +2H2O
(iii) NaCl +AgNO3 → AgCl +NaNO3
(iv) BaCl2 +H2SO4 → BaSO4 +2HCl
Ques 7: Write the balanced chemical equations for the following reactions.
(a) Calcium hydroxide + Carbon dioxide → Calcium carbonate+ Water
(b) Zinc + Silver nitrate → Zinc nitrate + Silver
(c) Aluminium + Copper chloride → Aluminium chloride + Copper
(d) Barium chloride + Potassium sulphate → Barium sulphate + Potassium chloride
Solution: The balanced chemical reactions are:
(a) Ca(OH)2 +CO2 → CaCO3 +H2O
(b) Zn +2AgNO3 → Zn(NO3)2 +2Ag
(c) 2Al +3CuCl2 → 2AlCl3 +3Cu
(d) BaCl2 +K2SO4 → BaSO4 +2KCl
Ques 8: Write the balanced chemical equation for the following and identify the type of reaction in each case.
(a)Potassium bromide (aq) +Barium iodide (aq) → Potassium iodide (aq) +Barium bromide(s)
(b) Zinc carbonate (s) → Zinc oxide (s) +Carbon dioxide (g)
(c) Hydrogen (g) +Chlorine (g) → Hydrogen chloride (g)
(d) Magnesium (s) +Hydrochloric acid (aq) → Magnesium chloride(aq) +Hydrogen (g)
Solution: The balanced chemical reactions along with the type of reaction are:
(a) 2KBr (aq) +BaI2 (aq) → 2KI (aq) +BaBr2 (s): It is Double displacement reaction
(a)Potassium bromide (aq) +Barium iodide (aq) → Potassium iodide (aq) +Barium bromide(s)
(b) Zinc carbonate (s) → Zinc oxide (s) +Carbon dioxide (g)
(c) Hydrogen (g) +Chlorine (g) → Hydrogen chloride (g)
(d) Magnesium (s) +Hydrochloric acid (aq) → Magnesium chloride(aq) +Hydrogen (g)
Solution: The balanced chemical reactions along with the type of reaction are:
(a) 2KBr (aq) +BaI2 (aq) → 2KI (aq) +BaBr2 (s): It is Double displacement reaction
(b) ZnCO3 (s) → ZnO (s) +CO2 (g): It is Decomposition reaction
(c) H2 (g) +Cl2 (g) → 2HCl (g): It is Combination reaction
(d) Mg (s) +2HCl (aq) → MgCl2 (aq) +H2 (g): It is Displacement Reaction
Ques 9: What does one mean by exothermic and endothermic reactions? Give examples.
Solution: The chemical reactions which release energy in the form of heat, light, or sound are called exothermic reactions.
(c) H2 (g) +Cl2 (g) → 2HCl (g): It is Combination reaction
(d) Mg (s) +2HCl (aq) → MgCl2 (aq) +H2 (g): It is Displacement Reaction
Ques 9: What does one mean by exothermic and endothermic reactions? Give examples.
Solution: The chemical reactions which release energy in the form of heat, light, or sound are called exothermic reactions.
Example: Reaction of Sodium and Chlorine to form table salt
Observation: Combination reactions are exothermic in nature.
On the other hand, the reactions which absorb energy or require energy in order to proceed are called endothermic reactions.
example: photosynthesis process in which the plants use the energy from the sun to convert carbon dioxide and water to glucose and oxygen.
Reaction is : 6CO2 (g) +6H2O (l) → C6H12O6 (aq) +6O2 (g)
On the other hand, the reactions which absorb energy or require energy in order to proceed are called endothermic reactions.
example: photosynthesis process in which the plants use the energy from the sun to convert carbon dioxide and water to glucose and oxygen.
Reaction is : 6CO2 (g) +6H2O (l) → C6H12O6 (aq) +6O2 (g)
Ques 10: Why is respiration considered an exothermic reaction? Explain.
Solution: Respiration is considered as an exothermic reaction because in respiration the oxidation of glucose takes place which produces a large amount of the heat energy.
The reaction that take place is:
C6H12O6 (aq) +6O2 (g) → 6CO2 (g) +6H2O (l) + Energy emit
Ques 11: Why are decomposition reactions called the opposite of combination reactions? Write equations for these reactions.
Solution: Decomposition reactions are the type of the reactions in which a compound breaks down to form two or more substances. But to break down a compound in these reactions, a source of energy is reqired to proceed. Thus, they are the exact opposite of combination reactions in which two or more substances combine to give a new substance with the release of energy.
Let's take an example:
Decomposition Reaction: AB + Energy → A+ B
Combination reaction:
A+ B →AB + Energy
Solution: Respiration is considered as an exothermic reaction because in respiration the oxidation of glucose takes place which produces a large amount of the heat energy.
The reaction that take place is:
C6H12O6 (aq) +6O2 (g) → 6CO2 (g) +6H2O (l) + Energy emit
Ques 11: Why are decomposition reactions called the opposite of combination reactions? Write equations for these reactions.
Solution: Decomposition reactions are the type of the reactions in which a compound breaks down to form two or more substances. But to break down a compound in these reactions, a source of energy is reqired to proceed. Thus, they are the exact opposite of combination reactions in which two or more substances combine to give a new substance with the release of energy.
Let's take an example:
Decomposition Reaction: AB + Energy → A+ B
Combination reaction:
A+ B →AB + Energy
Ques 12: Write one equation each for decomposition reactions where energy is supplied in the form of heat, light or electricity.
Solution:
(a) CaCO3(s)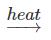 CaO(s) + CO2(g) {Heat Energy}
CaO(s) + CO2(g) {Heat Energy}
(b) 2AgCl(s)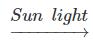 2Ag(s) + Cl2 (g) {Light Energy}
2Ag(s) + Cl2 (g) {Light Energy}
(c) 2H2O (l)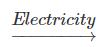 2H2(g) + O2(g) {Electricity}
2H2(g) + O2(g) {Electricity}
Solution:
(a) CaCO3(s)
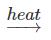 CaO(s) + CO2(g) {Heat Energy}
CaO(s) + CO2(g) {Heat Energy}(b) 2AgCl(s)
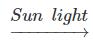 2Ag(s) + Cl2 (g) {Light Energy}
2Ag(s) + Cl2 (g) {Light Energy}(c) 2H2O (l)
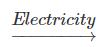 2H2(g) + O2(g) {Electricity}
2H2(g) + O2(g) {Electricity}Questions solutions of CBSE NCERT Class 10 Science on Page No: 16
Ques 13: What is the difference between displacement and double displacement reactions? Write equations for these reactions.
Solution: The
displacement reaction is a type of reaction in which a more reactive
element replaces a less reactive element from a compound.
A + BX→AX+ B
AB + CD→ AD+CB
A + BX→AX+ B
AB + CD→ AD+CB
where A is more reactive than B
The double displacement reaction is a type of reaction in which two atoms or a group of atoms switch places to form new compounds.
Example:
Displacement reaction:
CuSo4 (aq) +Zn (s) → ZnSO4 (aq) +Cu (s)
Double displacement reaction:
Na2SO4 (aq) +BaCl2 (aq) → BaSO4 (s) +2NaCl (aq)
Ques
14: In the refining of silver, the recovery of silver from silver
nitrate solution involved displacement by copper metal. Write down the
reaction involved.
Solution: The reaction involved in the above procedure is :
2AgNO3 (aq)+ Cu (s) → Cu(NO3)2 (aq) +2Ag (s)
OR, Silver Nitrate Copper → Copper Nitrate Silver
Ques 15: What do you mean by a precipitation reaction? Explain by giving examples.
Solution: The type of reaction in which an insoluble solid (called precipitate) is formed is called a precipitation reaction.
Example of precipitation reaction:Na2CO3 + CaCl2→CaCO3 + 2NaCl
In this reaction, calcium carbonate is obtained as a precipitate. Hence, it is a precipitation reaction.
Solution: The reaction involved in the above procedure is :
2AgNO3 (aq)+ Cu (s) → Cu(NO3)2 (aq) +2Ag (s)
OR, Silver Nitrate Copper → Copper Nitrate Silver
Ques 15: What do you mean by a precipitation reaction? Explain by giving examples.
Solution: The type of reaction in which an insoluble solid (called precipitate) is formed is called a precipitation reaction.
Example of precipitation reaction:Na2CO3 + CaCl2→CaCO3 + 2NaCl
In this reaction, calcium carbonate is obtained as a precipitate. Hence, it is a precipitation reaction.
Ques 16: Explain the following in terms of gain or loss of oxygen with two examples each.
(a) Oxidation
(b) Reduction
Solution:Oxidation Reaction:
Oxidation Reaction is a chemical reaction in which there is either gain of oxygen or loss of hydrogen.
(a) Oxidation
(b) Reduction
Solution:Oxidation Reaction:
Oxidation Reaction is a chemical reaction in which there is either gain of oxygen or loss of hydrogen.
Two examples of oxidation reaction are:
- 2Mg(s)+O2(g)→2MgO(s)
- 2Cu (s) + O2(g)→2CuO(s)
Reduction Reaction:
Reduction Reaction is a type of chemical reaction in which either there is loss of oxygen or gain of hydrogen.
Two examples of reduction reaction are:
Reduction Reaction is a type of chemical reaction in which either there is loss of oxygen or gain of hydrogen.
Two examples of reduction reaction are:
- CuO + H2→ Cu + H2O
- ZnO + C→ Zn + CO
Ques
17: A shiny brown-colored element ‘X’ on heating in air becomes black
in color. Name the element ‘X’ and the black colored compound formed.
Solution:The element asked ‘X’ is copper (Cu) and the black- colored compound that is formed is copper oxide (CuO).
Ques 18: Why do we apply paint on iron articles?
Solution:We apply paint on iron articles because paint prevents these from rusting. When the iron is painted, the contact of iron articles from moisture and air is cut off. Therefore, rusting is prevented. It should be noted that presence of air and moisture is essential for rusting to take place.
Ques 19: Oil and fat containing food items are flushed with nitrogen. Why?
Solution: As we know that, Nitrogen is an inert gas and it does not easily react with food substances. But on the other hand, oxygen reacts with food substances and makes them rancid. Therefore, the bags that are used in packing food items are flushed with nitrogen gas to remove oxygen filled inside the pack. And when the oxygen is not present inside the pack, rancidity of oil and fat containing food items is avoided.
Ques 20: Explain the following terms with one example each.
(a) Corrosion
(b) Rancidity
Solution: (a) Corrosion:
Corrosion is defined as the process in which the materials, usually metals, deteriorate or degrade as a result of a chemical reaction with air, moisture, chemicals, etc.
For example, iron, in the presence of moisture, reacts with oxygen to form hydrated iron oxide.
4Fe3O2 + nH2O → 2Fe2O3.nH2O
Solution:The element asked ‘X’ is copper (Cu) and the black- colored compound that is formed is copper oxide (CuO).
Ques 18: Why do we apply paint on iron articles?
Solution:We apply paint on iron articles because paint prevents these from rusting. When the iron is painted, the contact of iron articles from moisture and air is cut off. Therefore, rusting is prevented. It should be noted that presence of air and moisture is essential for rusting to take place.
Ques 19: Oil and fat containing food items are flushed with nitrogen. Why?
Solution: As we know that, Nitrogen is an inert gas and it does not easily react with food substances. But on the other hand, oxygen reacts with food substances and makes them rancid. Therefore, the bags that are used in packing food items are flushed with nitrogen gas to remove oxygen filled inside the pack. And when the oxygen is not present inside the pack, rancidity of oil and fat containing food items is avoided.
Ques 20: Explain the following terms with one example each.
(a) Corrosion
(b) Rancidity
Solution: (a) Corrosion:
Corrosion is defined as the process in which the materials, usually metals, deteriorate or degrade as a result of a chemical reaction with air, moisture, chemicals, etc.
For example, iron, in the presence of moisture, reacts with oxygen to form hydrated iron oxide.
4Fe3O2 + nH2O → 2Fe2O3.nH2O
NOTE:This hydrated iron oxide is known as rust.
(b) Rancidity:
The process of oxidation of fats and oils which can be easily noticed by the change in taste and smell is known as rancidity.
For example, the taste and smell of butter changes when kept for long.
Summing up CBSE NCERT solutions for Class 10 Science
How did you like the quality of the post "CBSE NCERT Class 10 Science Chapter 1 - Chemical Reactions and Equations solutions in english: TEGOFFICIAL.COM"?
Please tell your opinion about NCERT solutions for Class 10 Science in the comment box below.
Please don't forget to share the NCERT Class 10 Science solutions for free with your friends also and help them in their studies.
NCERT solutions for Class 10 Science pdf : Click on print button in options and save as pdf.
For more CBSE NCERT solutions for Class 10 Science keep visiting the website.
Until the next post,
For more CBSE NCERT solutions for Class 10 Science keep visiting the website.
Until the next post,
KEEP RISING...KEEP SMILING.
JAI HIND.
